Ultrahigh performance of a novel electrochemical deionization system based on a NaTi2(PO4)3/rGO nanocomposite†
Abstract
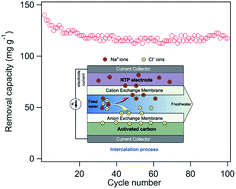
Capacitive deionization (CDI) has emerged as a simple, energy efficient, environment-friendly and cost effective technology for desalination of saline water in recent years. However, the salt removal capacity of CDI systems is not sufficient for desalting high-concentration seawater. Here we report a novel electrochemical deionization (EDI) system based on a NaTi2(PO4)3/rGO (NTP/rGO) composite, operated in a constant current mode. During the desalination process, sodium ions in the NaCl solution will be intercalated into the NTP/rGO electrode via a chemical reaction, while chloride ions are physically adsorbed on the other activated carbon (AC) electrode. The NTP/rGO based EDI system shows ultrahigh desalination performance. The salt removal capacity can reach 140 mg g−1 in the first cycle with a current density of 100 mA g−1, decreasing to as low as 120 mg g−1 after 100 cycles. Furthermore, a particularly rapid desalination rate of 0.45 mg g−1 s−1 has been achieved at 1000 mA g−1 with a removal capacity of 27 mg g−1. The excellent performance of the EDI system in this work has made it a promising desalination technology and provides great potential for direct seawater desalination in the future.


 Please wait while we load your content...
Please wait while we load your content...