Evolution of dealloyed PdBi2 nanoparticles as electrocatalysts with enhanced activity and remarkable durability in hydrogen evolution reactions†
Abstract
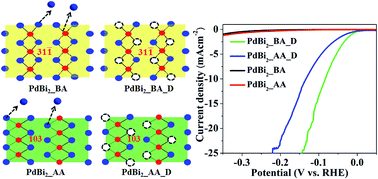
The development of non-Pt based electrocatalysts for the hydrogen evolution reaction (HER) is a pre-requisite for the generation of hydrogen, a feasible and cost-effective source of hydrogen. Structural transitions and generating metal deficiency are the effective ways of manipulating the d-band centre of a metal surface which enhances the catalytic activity of metal nanoparticles towards the HER. Charge-transfer from in situ generated oxide species to the metal centre also leads to an enhancement in catalytic activity towards the HER. In the present work, we report a facile colloidal synthesis of PdBi2 nanoparticles using sodium borohydride as the reducing agent. Upon annealing the as-synthesized nanoparticles, a phase transition from the lower symmetry monoclinic phase to the higher symmetry tetragonal phase has been observed, and hence a change in morphology from agglomerated to core–shell nanoparticles. Potential electrochemical cycling of both monoclinic and tetragonal PdBi2 leads to the formation of a Pd-rich PdBi2−x alloy with enhanced catalytic activity (onset potential: −11 mV and −18 mV vs. the RHE; 20 mA cm−2 current density at an overpotential of ∼140 mV and ∼207 mV for monoclinic and tetragonal phases, respectively). The low co-ordination number of Pd active sites formed by the dissolution of Bi alters the d-band centre of Pd, and hence the optimal energy required for hydrogen adsorption leading to enhanced activity. Although the obtained composition after potential cycling is almost similar for both phases it is seen that the dealloyed monoclinic phase shows higher activity as compared to the dealloyed tetragonal one. Cyclic voltammetry of the monoclinic PdBi2 shows the formation of Bi–O species after potential cycling. Electron transfer from the Bi–O species to the Pd centre enhances the charge-transfer kinetics of the HER on the catalyst surface and hence an increased catalytic activity of the monoclinic phase as compared to the tetragonal one. Thus, in situ generated oxide species facilitate charge-transfer from oxide to the metal surface which in turn enhances catalytic activity towards the HER.


 Please wait while we load your content...
Please wait while we load your content...