Nanoconfinement of redox reactions enables rapid zinc iodide energy storage with high efficiency†
Abstract
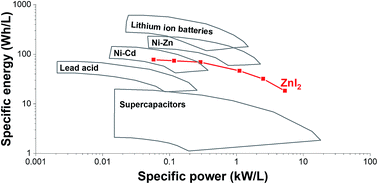
A key challenge for present-day electric energy storage systems, such as supercapacitors and batteries, is to meet the world's growing demand for high performances, low cost, and environmental-friendliness. Here, we introduce a hybrid energy storage system combining zinc iodide (ZnI2) as redox electrolyte with a nanoporous activated carbon fiber (ACF) cathode and a zinc disk anode. We found that the nanopores (<1 nm) of ACF lead to a strong adsorption behavior of iodide and triiodide. Hence, this system exhibits low self-discharge rates without applying an ion exchange membrane. The high power performance (20.0 kW kg−1) originates from the enhanced redox kinetics of the iodide system as evidenced by electrochemical analysis. Considering the high specific energy (226 W h kg−1), the ACF/Zn ZnI2 battery represents an alternative for lead acid, Ni–Zn, and Ni–Cd batteries, while providing a supercapacitor-like power performance in the range of seconds to minutes charging times.


 Please wait while we load your content...
Please wait while we load your content...