A rGO–CNT aerogel covalently bonded with a nitrogen-rich polymer as a polysulfide adsorptive cathode for high sulfur loading lithium sulfur batteries†
Abstract
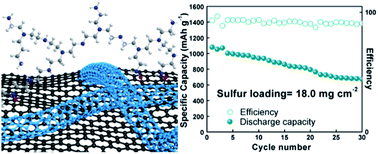
The development of high capacity lithium–sulfur (Li–S) batteries is hampered by both the shuttle effect of polysulfides and low sulfur areal loading problems. To inhibit the shuttle effect, a polysulfide adsorptive polymer polyethylenimine (PEI) is covalently bonded with reduced graphene oxide (rGO) in a one-step hydrothermal reaction. Meanwhile, multi-walled carbon nanotubes (MWCNTs) are simultaneously interweaved with rGO. The resultant PEI–rGO–MWCNT aerogel (PEI–GC) provides ample chemisorption domains of amine groups and abundant electrical contact sites. Density functional theory (DFT) calculations prove a binding energy of 2.43 eV between PEI and polysulfide. The PEI–GC cell achieved a high capacity of 933 mA h g−1 for the 500th cycle at 1C, stable rate performance up to 10C, and low self-discharge rate. The covalent bond between PEI and rGO experiences no degradation during the 500 cycles. Moreover, PEI–GC achieved excellent cycling performance at a high sulfur loading of up to 18 mg cm−2.


 Please wait while we load your content...
Please wait while we load your content...