Controllable synthesis of LiFePO4 in different polymorphs and study of the reaction mechanism†
Abstract
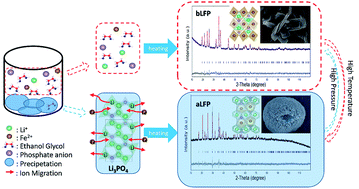
Lithium iron phosphate, a widely used cathode material in Lithium Ion Batteries (LIBs), crystallizes typically in an olivine-type phase, α-LiFePO4 (aLFP). However, the new phase β-LiFePO4 (bLFP), which can be transformed from aLFP at high temperature with high pressure, can be produced through a simple liquid-phase reaction. The mechanism of controllable synthesis of the two polymorphs of lithium iron phosphate has not been studied thoroughly. In this paper, with thorough experiments, we demonstrate that controllable synthesis of LFP with different crystal polymorphs can be obtained by controlling certain conditions. The phosphoric acid ratio in the reactants and the reaction time play key roles in the controllable syntheses. Higher phosphoric acid ratios and shorter reaction times would result in a higher bLFP content, while a lower amount of phosphoric acid and a longer reaction time would be beneficial to aLFP formation. To illustrate the mechanism for this phenomenon, the detailed reaction process was researched via X-ray diffraction, from which a possible mechanism associated with the evolution of crystal structures was demonstrated. The solvent content is also important for the process: some water content would lead to nanoplate-shaped aLFP particles appearing. Their influence on the reaction could be attributed to the change of thermodynamics and kinetics, which leads to different crystal nucleation, growth and phase-change processes.


 Please wait while we load your content...
Please wait while we load your content...