Computation-predicted, stable, and inexpensive single-atom nanocatalyst Pt@Mo2C – an important advanced material for H2 production†
Abstract
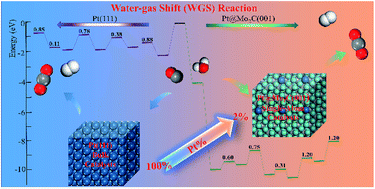
The finding that transition metals on Mo2C-supported nanocatalysts are promising for water-gas shift (WGS) reactions at room temperature has generated much excitement. However, the progress achieved with computational chemistry in this area is far behind that of experimental studies. Accordingly, density functional theory (DFT) calculations have been used to design the catalytic activity center structure and study the stabilities and catalytic performances of transition metals doped on β-Mo2C(001) surfaces. A new catalyst that comprises atomically dispersed Pt over Mo2C was designed using DFT. The bimetallic Mo2C surfaces doped with single metal Pt exhibit catalytic activities similar to those of the Pt systems for WGS, while demonstrating the advantages of lower costs and higher thermal stabilities. Importantly, the Pt@Mo2C catalyst is more efficient than the pure Pt catalyst for H2 production under the same reaction conditions. Meanwhile, the density of active sites of Pt@Mo2C(001) for H2 production is considerably increased due to its highly dispersed Pt structure. Therefore, Mo and Pt can synergistically increase H2 production. These findings are significantly beneficial for establishing the relationship between the structure and characteristics of the catalyst, understanding the catalytic activities of single-atom catalysts, and gaining insight into the feasibility of developing substitutes for expensive noble metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...