Novel biomolecule-assisted interlayer anion-controlled layered double hydroxide as an efficient sorbent for arsenate removal†
Abstract
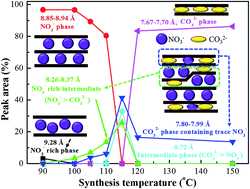
The synthesis of pure nitrate-containing layered double hydroxides (LDHs) via biomolecule-assisted methods is difficult to achieve without producing substantial waste. For the first time, we demonstrated the synthesis of LDHs with a controlled interlayer anion composition using an environmentally friendly L-arginine-assisted hydrothermal method with zero waste disposal. The mechanism of LDH formation was revealed through PXRD, FT-IR, XPS and ion chromatographic (IC) analyses. At low synthesis temperatures (90–110 °C), arginine-mediated water decomposition led to OH− and [Arg+]-NO3− formation and thus produced pure NO3−-containing LDHs. Conversely, at temperatures above 115 °C, L-arginine decomposition occurred and produced NH4+ and CO2, which resulted in CO32−-bearing LDHs. The FT-IR spectra of the solid residues, which were obtained at lower temperatures, indicated that several amino acids were functionalized on the surface of the LDHs and replaced by CO32−, which was produced at higher temperatures. The sorption of arsenate from an aqueous solution on the resulting LDHs showed maximum sorption capacity values of 1.675 and 1.972 mmol g−1 for Mg2.3Al-LDH and Mg2Al-LDH synthesised at 100 °C, respectively. The arsenate sorption capacity was enhanced by the functionalization of L-arginine compared with conventionally prepared LDHs. The mechanism of arsenate sorption was based on the ion-exchange of interlayer NO3− and functionalized arginine molecules. In summary, the chemical precursor L-arginine (utilized in this study) acts as a multifunctional reagent, including (i) a precipitant for the synthesis of LDH, (ii) an engineer for interlayer anion control, (iii) a functional reagent and (iv) a scavenger for free NO3− that is present in the synthesis medium. The current synthesis method did not utilize a hazardous base during synthesis, and the [Arg+]-NO3− byproduct can be used as a chemical source for health/skin care formulations with zero waste disposal, which offers great benefits.


 Please wait while we load your content...
Please wait while we load your content...