Tailored activated carbon from glycerol: role of acid dehydrator on physiochemical characteristics and adsorption performance†
Abstract
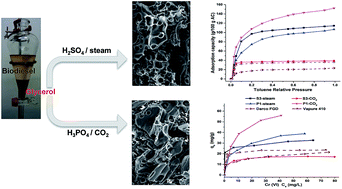
Glycerol, a by-product of biodiesel production, was systematically investigated as a liquid precursor for activated carbon (AC). In this study, acid-mediated dehydration, polymerization, and carbonization of liquid glycerol forms solid chars with unique surface textures and compositions. For the first time, a description of the dehydration acid's role in char formation is reported, relating decomposition mechanisms of H2SO4 and H3PO4 to resulting char properties. With this understanding, chars with variable functional groups and porosities are prepared based on distinguished acid–glycerol relationships. Porosity in chars is added with rapid physical activation, generating ACs with surface areas up to 2470 m2 g−1 and pore volumes up to 1.44 cm3 g−1. By combining different dehydration acids and activation agents (steam or CO2) with controlled pyrolysis/activation conditions, intentionally tailored ACs with variable surface areas, pore widths, structural configurations, and surface chemistries are obtained. To justify use, ACs are applied for removal of gas phase volatile organic compounds (VOCs) and aqueous phase chromium (Cr(VI)), outperforming commercial ACs. Toluene, hexane, and Cr(VI) adsorption capacities as high as 1.5 g g−1, 1.1 g g−1, and 56 mg g−1, respectively, are reported. Overall, glycerol is used to prepare AC with high and tuned porosity, and a facile method for producing tailored ACs from a liquid industrial waste is introduced.


 Please wait while we load your content...
Please wait while we load your content...