Oriented attachment growth of anisotropic meso/nanoscale MOFs: tunable surface area and CO2 separation†
Abstract
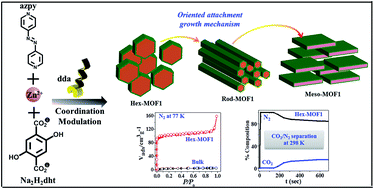
Scaling down bulk MOFs (metal–organic frameworks) to a nano/mesoscale is of paramount importance due to improved diffusion kinetics and surface area. To achieve this, a crystal growth mechanism and evolution of different multidimensional morphologies of MOFs in the nano/meso-regime need to be fully understood. Here, we report downsizing of a mixed-linker based 3D MOF to the nano/mesoscale by a coordination modulation method using n-dodecanoic acid as a modulator. We demonstrate changes in morphology and dimensionality (hexagonal nanoparticles → nanorods → mesosheets) by varying the modulator concentration. The time-dependent FESEM (field emission scanning electron microscopy) studies of the products obtained at different stages of synthesis suggest that the morphological transformation is driven by the kinetically controlled oriented attachment (OA) growth mechanism. Among various morphologies, hexagonal nanoparticles show a strikingly improved BET (Brunauer–Emmett–Teller) surface area compared to their bulk form due to a reduced diffusion barrier at the nanoscale as inferred from higher mass transfer kinetics. This is also reflected in better CO2/N2 separation efficiency in breakthrough column experiments at 298 K. This unprecedented on/off porosity with respect to N2 in nano vs. bulk-MOFs, respectively, is theoretically explained in terms of energetics of adsorption, atomic structural changes and electrostatic interactions between the MOF and adsorbate molecules.


 Please wait while we load your content...
Please wait while we load your content...