Bimetallic Ni–Mo nitride nanotubes as highly active and stable bifunctional electrocatalysts for full water splitting†
Abstract
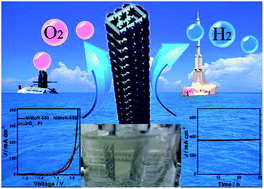
Designing low-cost, highly active and stable electrocatalysts is very important to various renewable energy storage and conversion devices. Herein we develop a facile method to fabricate bimetallic Ni–Mo nitride nanotubes, which can serve as highly active and stable bifunctional electrocatalysts for full water splitting. To drive a current density of 10 mA cm−2, the bimetallic Ni–Mo nitride nanotubes require an overpotential of 295 mV for the OER and 89 mV for the HER. The alkaline water electrolyzer with the nanotubes as cathode and anode catalysts requires a cell voltage of ca. 1.596 V to achieve a current density of 10 mA cm−2. Furthermore, the nanotubes for full water splitting show excellent stability even at a high current density of 370 mA cm−2, superior to the integrated performance of commercial Pt and IrO2. Our experimental results show that the NiOOH and NH groups formed at the catalyst surface during the OER process are active species for the OER, while the Ni(OH)2, NH and Mo species at the catalyst surface play a key role in the HER. The present strategy may open an avenue for fabrication of low-cost, highly active and stable electrocatalysts for large-scale water splitting.


 Please wait while we load your content...
Please wait while we load your content...