Morphology controlled synthesis of MnCO3–RGO materials and their supercapacitor applications†
Abstract
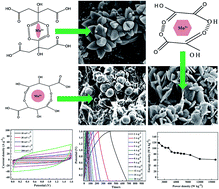
MnCO3-reduced graphene oxide (MnCO3–RGO) was grown on nickel foam by a facile successive ionic layer adsorption and reaction (SILAR) method and used as a supercapacitor electrode. The morphology of the MnCO3 functionalities was tuned from lotus to flake to spherical shape using different chelating agents during synthesis. The length and width of the individual petals of the lotus structure MnCO3 were found to be ∼200–300 and 50–100 nm, respectively. The reduction of graphene oxide (GO) in MnCO3–RGO composites was confirmed by Raman spectroscopy and electrical conductivity data analysis. The lotus shaped MnCO3 grown on RGO sheets provided a high surface area and electrical conductivity as compared to the developed electrode materials. The cyclic voltammetry, galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy analyses showed that the lotus shaped MnCO3 grown on RGO sheets provided higher current response, large specific capacitance (SC) and low solution, charge-transfer and Warburg resistance as compared to the flake and spherically shaped MnCO3 grown on RGO sheets. A fabricated asymmetric supercapacitor (ASC) device with MnCO3 (lotus) – RGO as the positive electrode and sonochemically reduced GO as the negative electrode – exhibited a working potential of ∼0–1.6 V, SC of ∼ 335 F g−1 at ∼2 A g−1 (∼468 mF cm−2 at ∼2.8 mA cm−2), an energy density of ∼120 W h kg−1 (∼0.16 mW h cm−2) and a power density of ∼16 kW kg−1 (∼22 mW cm−2) with a GCD stability of ∼73% after 10 000 cycles.


 Please wait while we load your content...
Please wait while we load your content...