Simple mono-halogenated perylene diimides as non-fullerene electron transporting materials in inverted perovskite solar cells with ZnO nanoparticle cathode buffer layers
Abstract
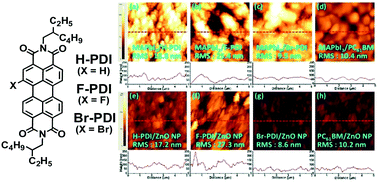
We have synthesized and characterized three perylene diimides, X-PDI, where X = H, F, or Br. These three compounds have been tested for the substitution of [6,6]-phenyl C61 butyric acid methyl ester (PC61BM) in inverted perovskite solar cells (PVSCs). Although the efficiency of PDI derivative-based conventional PVSCs is as high as 17.6% (J. Mater. Chem. A, 2016, 4, 8724), the corresponding performance of inverted PVSCs is still lagging behind and improvement is necessary. For electron accepting (from CH3NH3PbI3 perovskite) and electron transporting properties of the materials, UV-visible absorption spectroscopy, electrochemical cyclic voltammetry, direct current conductivity, space-charge limited current (SCLC) electron mobility, atomic force microscopy (AFM) surface morphology, and photoluminescence (PL) spectroscopy gauging charge-trapping have been studied. The HOMO/LUMO energy levels are 5.94/4.00, 5.83/3.96, 6.00/3.97, and 5.48/3.73 eV for H-PDI, F-PDI, Br-PDI, and PC61BM, respectively. Direct current conductivities of H-PDI, F-PDI, Br-PDI, and PC61BM are 8.71 × 10−8, 1.18 × 10−9, 2.2 × 10−6, and 8.42 × 10−6 S cm−1, respectively. The SCLC electron mobility of H-PDI, F-PDI, Br-PDI, and PC61BM are 1.12 × 10−4, 8.31 × 10−6, 1.08 × 10−3, and 5.00 × 10−3 S cm−1, respectively. PL spectroscopy of CH3NH3PbI3 perovskite provides emission at wavelength of 781 nm, which is the same as for the perovskite layer covered with a thin film of H-PDI or F-PDI. However, the emission wavelength was blue-shifted to 773–774 nm when the perovskite layer was covered with a thin film of Br-PDI or PC61BM. Using UV-visible absorption spectroscopy, the solubility (in chloroform) was determined as 1.2 × 10−2, 8.7 × 10−2 and >10−1 mol L−1 for F-PDI, H-PDI, and Br-PDI, respectively. In thin film state, UV-visible absorption spectroscopy indicated that the extent of molecular aggregation was F-PDI ≫ H-PDI > Br-PDI, which is consistent with the AFM-estimated root-mean-square roughness of F-PDI > H-PDI > Br-PDI ∼ PC61BM. Without the solution processed ZnO NP cathode buffer layer (CBL), the power conversion efficiency (PCE) of H-PDI, F-PDI, Br-PDI, and PC61BM PVSCs is ∼1%, ∼0%, 3.2%, and 4.1%, respectively. With the ZnO NP CBL, PCE is ∼7.8%, ∼0%, 10.5%, and 11.1% for H-PDI, F-PDI, Br-PDI, and PC61BM PVSCs, respectively. Through this study, we have demonstrated that the simple mono-bromine substituted perylene diimide (Br-PDI), is solution processable and has potential for use as a non-fullerene electron accepting and electron transporting material in inverted PVSCs.


 Please wait while we load your content...
Please wait while we load your content...