Facile synthesis of bowl-like 3D Mg(BH4)2–NaBH4–fluorographene composite with unexpected superior dehydrogenation performances†
Abstract
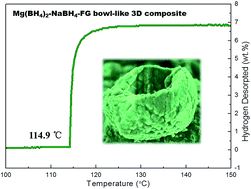
A remarkable improvement in the hydrogen desorption performance of Mg(BH4)2–NaBH4 eutectic composite is achieved by simply ball-milling with fluorographene (FG). It is found that the desorption temperature of Mg(BH4)2–NaBH4 (∼200 °C) is still too high and liquid phase appears during dehydrogenation. Particularly, the novel bowl-like 3D Mg(BH4)2–NaBH4–FG composite can be formed after ball-milling. The novel Mg(BH4)2–NaBH4–FG exhibits a low dehydrogenation temperature of 114.9 °C with 6.9 wt% of pure hydrogen in seconds. In-depth investigations show that such greatly improved hydrogen desorption properties of the Mg(BH4)2–NaBH4–FG composite could be ascribed to both the “novel bowl-like 3D structure” with large specific surface area and the “reactant destabilized modification” owing to the decrease of the reaction enthalpy caused by the formation of NaMgF3. This finding provides a facile preparation of complex borohydride composites with low dehydrogenation temperature and fast rate, which accelerates its practical application in fuel cells.


 Please wait while we load your content...
Please wait while we load your content...