Electron acceptors with varied linkages between perylene diimide and benzotrithiophene for efficient fullerene-free solar cells†
Abstract
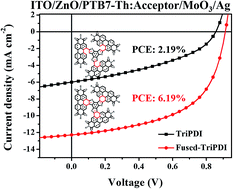
In this work, we present two new electron acceptors, TriPDI and Fused-TriPDI, in which three perylene diimide (PDI) moieties are tethered to a benzotrithiophene (BTT) core via either single bonds (TriPDI) or ring-fusion (Fused-TriPDI). TriPDI connects three PDIs to BTT via carbon–carbon single bonds, resulting in a rotatable and highly twisted skeleton. Instead, Fused-TriPDI, made through oxidative ring-fusion of TriPDI, exhibits good structural rigidity and planarity, as well as effective conjugation between PDI and BTT. As a result, the fused molecule shows up-shifted energy levels, and enhanced absorption and charge mobility over the unfused one. The polymer solar cells (PSCs) with a PTB7-Th:Fused-TriPDI blend show the best power conversion efficiency of 6.19%, which is around three times higher than that with PTB7-Th:TriPDI.


 Please wait while we load your content...
Please wait while we load your content...