Achieving over 10% efficiency in a new acceptor ITTC and its blends with hexafluoroquinoxaline based polymers†
Abstract
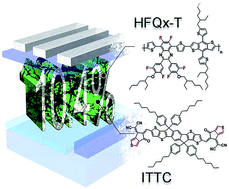
A new small molecule, ITTC, bearing an indacenodithieno[3,2-b]thiophene core and a 2-(6-oxo-5,6-dihydro-4H-cyclopenta[c]thiophen-4-ylidene)malononitrile end group, was designed, synthesized and characterized as a non-fullerene electron acceptor. The ITTC possesses strong and broad light absorption, high and balanced charge mobility and a nanoscale interpenetrating morphology when blended with a recently synthesized hexafluoroquinoxaline based polymer donor-HFQx-T. HFQx-T was obtained from a Stille coupling copolymerization of a 2,6-bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)thiophene-2-yl)benzo[1,2-b:4,5-b′]dithiophene electron donating unit and a hexafluoroquinoxaline based electron accepting unit. The device employing HFQx-T as the donor and ITTC as the acceptor delivered a power conversion efficiency (PCE) of 8.19% without any post-treatment. After thermal annealing, an impressive PCE of 10.4% was obtained. This performance is among the highest PCEs reported for fullerene-free polymer solar cells up to date. This study demonstrates the great potential of ITTC as n-type materials for organic electronics.


 Please wait while we load your content...
Please wait while we load your content...