Biomimetic reduction of O2 in an acid medium on iron phthalocyanines axially coordinated to pyridine anchored on carbon nanotubes
Abstract
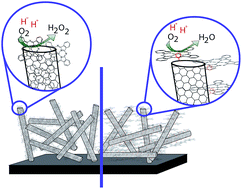
An efficient and inexpensive catalyst for the oxygen reduction reaction (ORR) is the key missing component for large-scale development of fuel cells. Bio-inspired tethered electrocatalysts could be the solution to this problematic reaction. Either unsubstituted Fe phthalocyanine (FePc) or Fe hexadecachloro-phthalocyanine (16(Cl)FePc) was anchored to carbon nanotubes (CNTs) via a pyridine axial ligand. The results show that the fifth coordination plays a major role in increasing the catalytic activity of FePc and 16(Cl)FePc for the ORR. The coordination also allows the decoupling of the metal centre from the carbon support, thus changing the geometrical and electronic structure and hindering the production of H2O2. The pentacoordinated catalysts were stable in acidic pH according to the rotating disk analysis, but the activity of the hexadecachloro compound was not higher than that of the unsubstituted phthalocyanine. Cl atoms reduced the coupling between O2 and Fe, mismatching the energy of the frontier orbitals and lowering the activity towards the reduction of O2.


 Please wait while we load your content...
Please wait while we load your content...