In situ formation of a 3D core/shell structured Ni3N@Ni–Bi nanosheet array: an efficient non-noble-metal bifunctional electrocatalyst toward full water splitting under near-neutral conditions†
Abstract
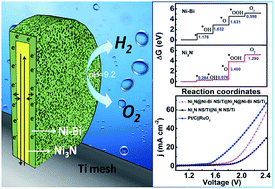
It is of great importance but still remains a key challenge to develop non-noble-metal bifunctional catalysts for efficient full water splitting under mild pH conditions. In this communication, we report the in situ electrochemical development of an ultrathin Ni–Bi layer on a metallic Ni3N nanosheet array supported on a Ti mesh (Ni3N@Ni–Bi NS/Ti) as a durable 3D core/shell structured nanoarray electrocatalyst for water oxidation at near-neutral pH. The Ni3N@Ni–Bi NS/Ti demands overpotentials of 405 and 382 mV to deliver a geometrical catalytic current density of 10 mA cm−2 in 0.1 and 0.5 M K–Bi (pH: 9.2), respectively, superior in activity to Ni3N NS/Ti and most reported non-precious metal catalysts under benign conditions. It also performs efficiently for the hydrogen evolution reaction requiring an overpotential of 265 mV for 10 mA cm−2 and its two-electrode electrolyser affords 10 mA cm−2 at a cell voltage of 1.95 V in 0.5 M K–Bi at 25 °C.


 Please wait while we load your content...
Please wait while we load your content...