Bioinspired electrocatalysts for oxygen reduction using recombinant silk films†
Abstract
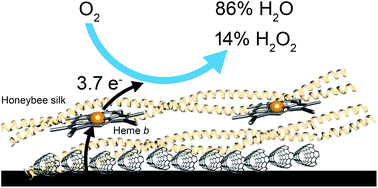
Fuel cells are a promising avenue for renewable energy production. While oxygen remains the preferred oxidant, its slow reduction kinetics has limited fuel cell performance and it currently requires the use of platinum as the cathode catalyst. In the search for non-platinum cathodes, inspiration has been sought from biological oxygen reduction processes which use heme proteins for respiration. Here, we describe the use of recombinant honeybee silk protein, which can be produced at high scale in E. coli, to generate a heme–protein material. In these solid-state silk materials, a tyrosine residue coordinates directly to the heme iron center. This axial coordination promotes heterolytic O–O bond cleavage, rather than homolytic cleavage, avoiding the generation of destructive hydroxyl radicals. The heme–silk materials can fully reduce oxygen to water with 3.7 electrons transferred to oxygen and only 14% hydrogen peroxide produced. Importantly, the films demonstrate remarkable stability. The films retained activity when used under continuous operation for over 16 hours and retained 85% of their catalytic activity when used at pH 3 for two hours.


 Please wait while we load your content...
Please wait while we load your content...