One-electron intermediates of water oxidation & the role of solvation in their stability
Abstract
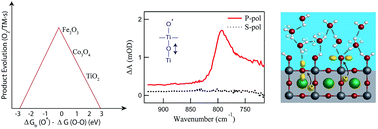
The initial step of the electrochemically controlled water oxidation reaction on transition metal oxide surfaces localizes positive charge onto surface atoms, creating one-electron intermediates. For decades, the stability of this critical reaction intermediate has been used to predict catalytic activity, by a Sabatier analysis of the first bond (O–O) the intermediates catalyse within the O2 evolution cycle. Recently, several groups directly detected these intermediates by their specific vibrations during the reaction on different material surfaces (Co3O4, Fe2O3, SrTiO3). By observing surface bound one-electron intermediates, these experiments validate a Sabatier analysis. However, the experiments strongly suggest that solvation by the electrolyte is important to their stability, which has not been a focus of calculations of their charge trapping capacity. For the time-resolved (femtosecond) experiments of the SrTiO3 surface, solvation by water rate limits the intermediates' formation time constant. Further, the kinetic stability or lifetime of the intermediates across diverse material surfaces points to the importance of re-organization within the electrolyte. In this highlight, we review these recent results, put them in the context of theoretical investigations, and discuss the implications of solvation-enabled intermediate stability for probing and understanding catalytic mechanism at surfaces.
- This article is part of the themed collections: Recent Review Articles and Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...