Combination of four oxadiazole rings for the generation of energetic materials with high detonation performance, low sensitivity and excellent thermal stability†
Abstract
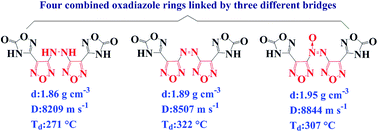
Energetic materials, which are comprised of four oxadiazole rings and linked by three different bridges ([–NH–NH–], [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–], and [–N
N–], and [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(O)–]) are developed. All synthesized compounds were fully characterized and five of them were further determined by single-crystal X-ray diffraction. As supported by X-ray data, closed packing and extensive hydrogen-bonding interactions result in high density, low sensitivity, and excellent thermal stability. It is worth pointing out that the [–N
N(O)–]) are developed. All synthesized compounds were fully characterized and five of them were further determined by single-crystal X-ray diffraction. As supported by X-ray data, closed packing and extensive hydrogen-bonding interactions result in high density, low sensitivity, and excellent thermal stability. It is worth pointing out that the [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–] compound 3 has a decomposition temperature of 322 °C, which, to our knowledge, is the highest known value for all compounds consisting of 1,2,4- and 1,2,5-oxadiazole rings. Dihydrazinium salt 14 exhibits excellent detonation performance (D = 9042 m s−1, P = 35.0 GPa, and IS > 40 J), superior even to RDX. This novel design strategy, which combines four oxadiazole rings into one molecule, promises a fine balance between high detonation performance and low sensitivity and opens a new chapter in oxadiazole chemistry.
N–] compound 3 has a decomposition temperature of 322 °C, which, to our knowledge, is the highest known value for all compounds consisting of 1,2,4- and 1,2,5-oxadiazole rings. Dihydrazinium salt 14 exhibits excellent detonation performance (D = 9042 m s−1, P = 35.0 GPa, and IS > 40 J), superior even to RDX. This novel design strategy, which combines four oxadiazole rings into one molecule, promises a fine balance between high detonation performance and low sensitivity and opens a new chapter in oxadiazole chemistry.


 Please wait while we load your content...
Please wait while we load your content...