Transparent flexible lithium ion conducting solid polymer electrolyte†
Abstract
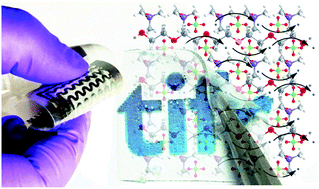
Recent safety threats concerning conventional liquid electrolyte-based Li-ion batteries invoke the search for high ionic conductivity solid electrolytes (SEs) for solid state batteries. Here, the development of a multifunctional polymer SE (ionic conductivity ∼0.03 mS cm−1) is demonstrated and this SE is endowed with other exotic properties such as high Li-ion transport number (∼0.69) with large electrochemical window (2–5 V), high mechanical robustness and flexibility (Young's modulus ∼1 MPa), visible light transparency (∼85%), and hydrophobicity (contact angle > 100°). Here poly(ethylene oxide) (PEO) and polydimethylsiloxane (PDMS) based polymer complex serves as the Li-ion transport membrane, and lithium perchlorate (LiClO4) as the Li source. The ‘salting in’ phenomenon, induced by the ClO4−–PEO interactions, modifies the crystalline melting temperature of PEO leading to the amorphization of the PEO–PDMS matrix and hence to a high Li-ion conductivity by microstructure modifications. This transparent and flexible SE is shown for its applicability in flexible symmetric capacitors and Li-ion cells without the use of liquid electrolyte interfaces.


 Please wait while we load your content...
Please wait while we load your content...