A general approach to synthesise ultrathin NiM (M = Fe, Co, Mn) hydroxide nanosheets as high-performance low-cost electrocatalysts for overall water splitting†
Abstract
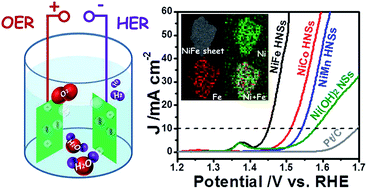
Electrochemically splitting water into hydrogen (H2) and oxygen (O2) is a promising method for clean energy generation, while the absence of highly active, stable, low-cost and earth abundant catalysts greatly impedes its large-scale application. Herein, we report a general and robust approach for the controlled synthesis of a class of NiM (M = Fe, Co, Mn) hydroxide nanosheets (HNSs) that have ultrathin thicknesses of around 2 nm. Such unique structures enable the HNSs to have promising oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) performances, with the NiFe HNSs being the best candidate. Given the well-defined electrochemical bifunctionality, a full alkaline electrolyzer was constructed using NiFe HNSs as both the cathodic and the anodic catalysts. It can realize overall water splitting with a current density of 10 mA cm−2 at 1.67 V and has remarkable durability for 12 h. This work opens a new avenue to approach water splitting catalysis using efficient low-cost Ni-based HNSs.


 Please wait while we load your content...
Please wait while we load your content...