Design principles of perovskites for solar-driven thermochemical splitting of CO2†
Abstract
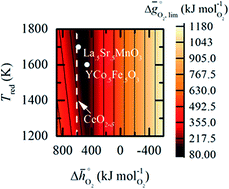
Perovskites are attractive redox materials for thermo/electrochemical fuel synthesis. To design perovskites with balanced redox energetics for thermochemically splitting CO2, the activity of lattice oxygen vacancies and stability against crystal phase changes and detrimental carbonate formation are predicted for a representative range of perovskites by electronic structure computations. Systematic trends in these materials properties when doping with selected metal cations are described in the free energy range defined for isothermal and temperature-swing redox cycles. To confirm that the predicted materials properties root in the bulk chemical composition, selected perovskites are synthesized and characterized by X-ray diffraction, transmission electron microscopy, and thermogravimetric analysis. On one hand, due to the oxidation equilibrium, none of the investigated compositions outperforms non-stoichiometric ceria – the benchmark redox material for CO2 splitting with temperature-swings in the range of 800–1500 °C. On the other hand, certain promising perovskites remain redox-active at relatively low oxide reduction temperatures at which ceria is redox-inactive. This trade-off in the redox energetics is established for YFeO3, YCo0.5Fe0.5O3 and LaFe0.5Ni0.5O3, identified as stable against phase changes and capable to convert CO2 to CO at 600 °C and 10 mbar CO in CO2, and to being decomposed at 1400 °C and 0.1 mbar O2 with an enthalpy change of 440–630 kJ mol−1 O2.


 Please wait while we load your content...
Please wait while we load your content...