Atomistic insight into the electrode reaction mechanism of the cathode in molten carbonate fuel cells†
Abstract
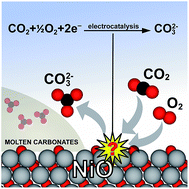
In an era of increasing energy demand challenges combined with simultaneous environmental protection, molten carbonate fuel cells (MCFCs) have emerged as an interesting candidate to overcome both of these issues. Although the macroscopic parameters of MCFCs have been successfully optimized, the microscopic understanding of the electrochemical catalytic reactions, which determine their performance, remains challenging due to their chemical complexity and high operation temperatures. In this paper, we propose a top-down approach to unravel the hitherto unreported electrode reaction mechanism of the cathode in MCFCs using density functional theory (DFT). The oxygen-terminated octopolar NiO(111) is predicted to facilitate cathodic transformation of carbon dioxide to carbonate anions through sequential Mars-van Krevelen (MvK) and Eley-Rideal (ER) mechanisms. This theoretical work opens up new prospects in the atomic scale computational design of the cathode material for MCFCs.


 Please wait while we load your content...
Please wait while we load your content...