MgCl2/AlCl3 electrolytes for reversible Mg deposition/stripping: electrochemical conditioning or not?†
Abstract
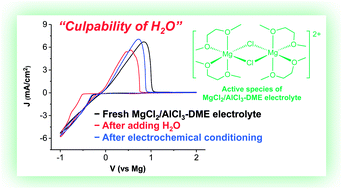
To elucidate the discrepancy of electrochemical performance of inorganic MgCl2/AlCl3 electrolytes in ethereal solvents, we conducted a systematic study of MgCl2/AlCl3–DME electrolytes under different conditions. Under optimized conditions, the 1 : 1 MgCl2/AlCl3 electrolyte in vigorously dried 1,2-dimethoxyethane (DME) showed unprecedented performance regarding Mg deposition/stripping including reversibility (up to 100% CE), electrolyte conductivity (>8.5 mS cm−1), and anodic stability (3.5 V vs. Mg). We showed that water impurity could jeopardize the electrochemical performance of the electrolytes, and the adverse influence caused by water could be recovered through continuous cyclic voltammogram scans, previously known as electrochemical conditioning. The results indicate that the cause of the electrochemical conditioning in previous studies is attributed to the high water level present in electrolytes and highlights that it is critical to carefully control experimental conditions to yield reliable data for Mg electrolytes. A comprehensive discussion of coordination chemistry on MgCl2/AlCl3–DME electrolytes is also present.


 Please wait while we load your content...
Please wait while we load your content...