Effect of alkaline-earth species in phosphate glasses on the mobility of proton carriers†
Abstract
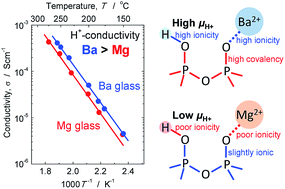
In this work, the effect of alkaline-earth species used in phosphate glasses on the mobility of proton carriers was investigated. Initially, 35NaO1/2–5RO–3NbO5/2–3LaO3/2–2GeO2–2BO3/2–50PO5/2 (R: Mg and Ba) glasses were subjected to the electrochemical substitution of Na+ ions with H+ at intermediate temperatures, producing proton conductors with high proton carrier concentrations (>8 × 1021 cm−3). The mobility of the proton carriers in the Ba glass was higher than that in the Mg glass. We studied the origin of this phenomenon in terms of the O–H and P–O bonding features using infrared spectroscopy and X-ray photoelectron spectroscopy, respectively. The higher mobility of proton carriers in Ba glass was attributed to weaker O–H bonding, which originated from the highly ionic character of Ba–O bonding compared with the comparatively covalent Mg–O bonding. Our findings indicate that a higher mobility of proton carriers can be achieved for glasses containing less electronegative glass network modifiers. The application of this strategy would be beneficial for the development of proton-conducting glass electrolytes for intermediate-temperature fuel cells.


 Please wait while we load your content...
Please wait while we load your content...