Balancing the chemisorption and charge transport properties of the interlayer in lithium–sulfur batteries†
Abstract
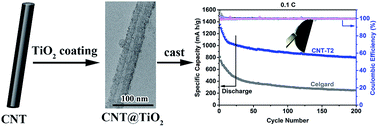
This study introduces an improved design of the interlayer between the cathode and separator of rechargeable lithium–sulfur batteries to mitigate the polysulfide crossover problem of the latter. The design involves integrating carbon nanotubes with titanium dioxide by a facile room-temperature hydrolytic method to form a titanium dioxide coated carbon nanotube composite (CNT@TiO2) with customizable TiO2 content. The CNT@TiO2 composite was then coated on a separator to form an interlayer much thinner than other standalone interlayers. The TiO2 coating on the CNT surface provides the facility for lithium polysulfides (LiPS) interception by chemisorption, and the underlying CNT core renders the intercepted LiPS electrochemically viable in charging and discharging. A good balance between the chemisorption properties of TiO2 and the charge transport properties of the CNTs is required to deliver a good interlayer performance because of the complementarity of these functions. Consequently, a battery with an optimized CNT@TiO2 interlayer composition could deliver a high initial capacity of 1351 mA h g−1 and a discharge capacity of 803 mA h g−1 after 200 cycles at 0.1C, for less than half of the thickness of a typical standalone interlayer (12 μm).


 Please wait while we load your content...
Please wait while we load your content...