Dimensionally stable hexamethylenetetramine functionalized polysulfone anion exchange membranes†
Abstract
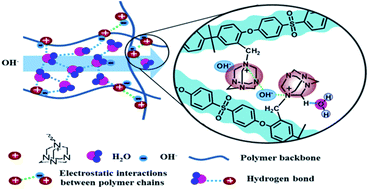
A novel alkaline group, hexamethylenetetramine (HMTA) with four tertiary amine groups and β-hydrogen-absent structure, has been employed as mono-quaternization reagent to prepare HMTA mono-quaternized polysulfone anion exchange membranes (PSF-QuOH AEMs). Analyzed by molecular dynamics simulations, mono-quaternized HMTA shows a superior aggregating ability by strong electrostatic interaction with hydroxide to suppress water swelling even at high IECs. In particular, the PSF-QuOH membrane with a high IEC of 2.23 mmol g−1 exhibits a low swelling ratio of 21% even at 60 °C. The resulting high concentration of cationic groups and the interactions between multiple hydrogen atoms in HMTA and hydroxide/water are helpful to induce the formation of the continuous and efficient hydrogen-bond networks, promoting ionic transport. High hydroxide conductivity of 35 mS cm−1 is achieved at 20 °C. Excellent swelling resistance also benefits the mechanical and chemical stabilities of the PSF-QuOH membranes. A considerable mechanical strength of 17.7 MPa is observed in the fully hydrated membrane. The hydroxide conductivity is stable at around 86% of the initial value after 1 M KOH immersion at 60 °C for 168 h.


 Please wait while we load your content...
Please wait while we load your content...