Enhanced proton conduction by post-synthetic covalent modification in a porous covalent framework†
Abstract
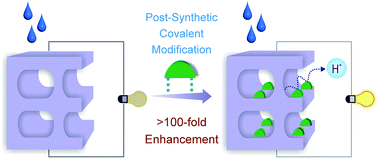
A highly chemically stable porous covalent framework (PCF-1) based on ether linkages has been synthesized, which exhibits no loss up to ∼500 °C along with retention of integrity under acidic, basic and oxidative reagent conditions. Owing to its thermal and chemical stability, post-synthetic covalent modification was executed for the introduction of pendant sulphonic acid (–SO3H) groups. The covalently modified compound (PCF-1-SO3H) presents a remarkably high conductivity (ca. 0.026 S cm−1), with an ∼130 fold enhancement in proton conductivity over the parent compound. This value is comparable with those of commercially used Nafion-based proton conducting materials and stands as the highest known value in the regime of post-synthetically modified porous organic frameworks. It is noteworthy to mention that PCF-1 is stable in both acidic and alkaline media, which is not commonly observed for most of the porous materials trialed as proton conducting materials, including metal–organic frameworks.


 Please wait while we load your content...
Please wait while we load your content...