Redox chemistry of CaMnO3 and Ca0.8Sr0.2MnO3 oxygen storage perovskites†
Abstract
Perovskite oxides CaMnO3 and Ca0.8Sr0.2MnO3 show continuous non-stoichiometry over a range of temperatures and oxygen partial pressures. In this work a thermobalance equipped with an oxygen pump was used to measure the equilibrium non-stoichiometry of both materials for temperatures in the range 400–1200 °C and oxygen partial pressures in the range 1–10−5 bar. Analysis of the data showed that Ca0.8Sr0.2MnO3 has a lower enthalpy of reduction and thus can be more easily reduced. The strontium added sample was also robust against a phase transition that was seen in CaMnO3 at high temperatures. A statistical thermodynamic model of the system suggests that the defects form clusters of the form 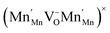 . The oxidation kinetics were also investigated with Ca0.8Sr0.2MnO3 showing faster kinetics and maintaining activity at lower temperatures. Overall, Ca0.8Sr0.2MnO3 shows very promising properties for redox applications, including gravimetric oxygen storage up to 4% by mass, high stability and rapid reversibility, with re-oxidation in less than 1 min at 400 °C. Finally, the redox chemistry of Ca0.8Sr0.2MnO3 was also investigated using in situ X-ray photoelectron spectroscopy and near-edge X-ray absorption measurements at near ambient pressure in oxygen atmospheres.
. The oxidation kinetics were also investigated with Ca0.8Sr0.2MnO3 showing faster kinetics and maintaining activity at lower temperatures. Overall, Ca0.8Sr0.2MnO3 shows very promising properties for redox applications, including gravimetric oxygen storage up to 4% by mass, high stability and rapid reversibility, with re-oxidation in less than 1 min at 400 °C. Finally, the redox chemistry of Ca0.8Sr0.2MnO3 was also investigated using in situ X-ray photoelectron spectroscopy and near-edge X-ray absorption measurements at near ambient pressure in oxygen atmospheres.


 Please wait while we load your content...
Please wait while we load your content...