Side chain engineering of organic sensitizers for dye-sensitized solar cells: a strategy to improve performances and stability†
Abstract
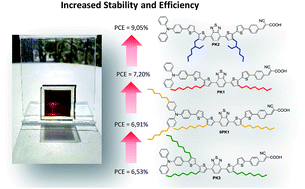
Four organic dyes specifically designed for application in dye-sensitized solar cells are reported. These dyes are based on a perfectly identical π-conjugated backbone and only differ by the number and the nature of the alkyl chain substituents employed as solubilizing groups. These sensitizers have outstanding light absorption properties in the visible range up to 750 nm on TiO2 and they exhibit quite similar energy level positions. Solar cells fabricated and characterized under exactly the same conditions with iodine-based liquid electrolytes show performances ranging from 6.53% to 9.05%. We highlight that the nature and the number of solubilizing groups have a tremendous impact on the performances of solar cells. The differences in the performance of the four sensitizers can be correlated with the current generation, the formation of aggregates and the dye-loading on the electrodes. We also report solar cells with ionic liquid electrolytes that demonstrate power conversion efficiencies up to 7.81% and a good stability of the performances under accelerated ageing conditions for 7300 hours. We establish that the number and the type of solubilizing groups attached on the π-conjugated backbone of the dyes have a strong influence not only on the performances but also on the lifetimes of the devices; i.e. the dyes that contain lower ratios of alkyl groups lead to the more stable cells.


 Please wait while we load your content...
Please wait while we load your content...