Enhancement of the H2 evolution activity of La5Ti2Cu(S1−xSex)5O7 photocatalysts by coloading Pt and NiS cocatalysts†
Abstract
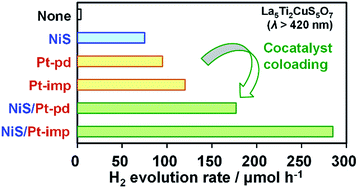
Enhancing the activity of narrow bandgap semiconductor photocatalysts is an essential step toward realizing efficient sunlight-driven water splitting for renewable H2 production. La5Ti2Cu(S1−xSex)5O7 (LTCS1−xSexO, 0 ≤ x ≤ 0.6) solid solutions are considered potential candidates for photocatalysts owing to their ability to absorb visible light with wavelengths of up to 650–780 nm and to evolve H2 from aqueous solutions via both photocatalytically and photoelectrochemically. In the present work, LTCS1−xSexO (0 ≤ x ≤ 0.6) solid solution photocatalysts coloaded with Pt and NiS cocatalysts exhibited higher activity than those loaded with Pt or NiS alone during the H2 evolution reaction from aqueous solutions containing Na2S and Na2SO3. The apparent quantum efficiency of LTCSO (x = 0) loaded with Pt and NiS was 1.8% at 420 ± 10 nm, a value that is three times higher than that for LTCSO loaded with NiS alone. The roles of the cocatalysts in the H2 evolution reaction were studied by assessing photocathodes and photoanodes composed of LTCSO loaded with Pt and/or NiS. It is suggested that Pt effectively facilitates the water reduction reaction while NiS enhances both H2 evolution and the oxidation of the sacrificial reagents. The ability of the coloaded cocatalysts to promote both the reduction and oxidation processes is considered to be of particular importance for the activation of narrow-bandgap photocatalysts with relatively low reactivities.


 Please wait while we load your content...
Please wait while we load your content...