Enhancing the oxygen vacancy formation and migration in bulk chromium(iii) oxide by alkali metal doping: a change from isotropic to anisotropic oxygen diffusion†
Abstract
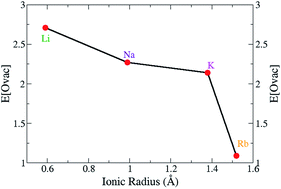
Oxygen vacancy formation and migration are vital properties for reducible oxides such as TiO2, CeO2 and Cr2O3 as the oxygen storage capacity (OSC) of these materials are important for a wide range of applications in photovoltaics, oxidative catalysis and solid oxide fuel cells. Substitutional doping these transition metal oxides enhances their OSC potential, in particular for oxygenation and surface reaction chemistry. This study uses density functional theory with on-site Coulomb interactions (PBE+U) for Cr 3d states (+U = 5 eV) and O 2p states (+U = 5.5 eV) to calculate the oxygen vacancy formation energy and oxygen diffusion pathways for alkali metal (Li, K, Na, Rb) doping of bulk chromium(III) oxide (α-Cr2O3). Substitutional doping of the lattice Cr3+ cations with alkali metals that have a +1 oxidation state, creates two hole states on the neighbouring lattice O atoms, and removal of a lattice oxygen charge compensates the dopants by filling the holes. The removal of the next oxygen describes the reducibility of doped Cr2O3. The oxygen vacancy formation energy is greatly promoted by the alkali dopants with a correlation between the ionic radius of the dopant cation and vacancy formation energy; larger dopants (K, Rb) improve the reducibility more than the smaller dopants (Li, Na). The activation barriers for oxygen migration along different directions in the alkali metal doped Cr2O3 bulk were also calculated to examine the effect of doping on the oxygen migration. The calculated activation energies for the undoped chromia are symmetric in three dimensions (isotropic) and the presence of the dopants break this isotropy. Alkali dopants promote oxygen migration in the oxygen intra-layers while suppressing oxygen migration across the Cr cation layers. The smaller dopants (Li, Na) facilitate easier migration in the oxygen intra-layers to a greater extent than the larger dopants (K, Rb). The Na–Cr2O3 bulk promotes both oxygen vacancy formation and migration which makes it a novel candidate for anode materials in medium temperature SOFCs and battery applications.


 Please wait while we load your content...
Please wait while we load your content...