Effective charge-discriminated group separation of metal ions under highly acidic conditions using nanodiamond-pillared graphene oxide membrane†
Abstract
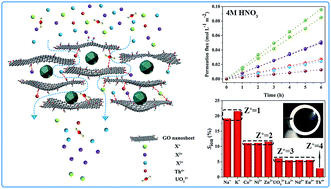
It has been a major challenge for separation science to separate selectively valuable metal ions from multi-cation aqueous solutions, especially in highly acidic media. In this study, a novel nanodiamond-pillared graphene oxide (NPG) membrane was, for the first time, prepared via intercalation of nanodiamond (ND) into graphene oxide (GO) by using a simple vacuum-assisted self-assembly method. Comprehensive characterization and comparison of GO and its derivative membranes show that after intercalation of ND into GO, the interlayer space and intrastratal nanochannels (similar to “the number of theoretical plates” in chromatography) are markedly increased; the channel-size distribution is profitably narrowed, and the thermal stability and hydrophilicity are enhanced; distinctively, the surface microroughness is significantly improved. The results of ion-sieving experiments demonstrate that the NPG nanofiltration membrane possesses a precise charge-discriminated group separation ability in a strong acidic solution containing 10 coexisting cations. Compared with the unmodified GO membrane, the average filtration rates of the NPG membrane were improved by 76.8% (for monovalent ions), 63.5% (for divalent ions), 118.0% (for trivalent lanthanide ions), 71.0% (for UO22+) and 105.1% (for Th4+) respectively, under the same conditions, meanwhile, the permeation rate of the multivalent cations with same charges shows much better consistency. It’s worth mentioning that UO22+ has the largest hydrated diameter among the cations used in this work, but the filtration rate of UO22+ is actually similar to that of the trivalent lanthanide cations, which may be attributed to the effective charge near +3 of UO22+, as reported in the literature. Finally, the reusability of the as-prepared nanofiltration membrane was investigated in 4 M nitric acid solution. After 5 runs of recycling tests, the group separation ability of the NPG membrane was basically maintained, and the filtration rate was reduced by up to 15.2% (for K+). These findings suggest that the as-prepared carbonaceous nanofiltration membrane could be among the most attractive membrane materials for the separation of valuable metal ions and have great potential applications in not only the nuclear power industry, but also the metallurgical, chemical manufacturing, electroplating and metal finishing industries.


 Please wait while we load your content...
Please wait while we load your content...