Enhanced storage of sodium ions in Prussian blue cathode material through nickel doping†
Abstract
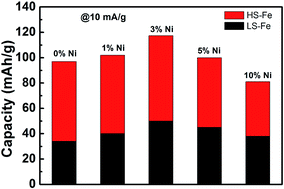
Prussian blue (PB) is a promising and cost-effective material for sodium ion batteries (SIBs) because it possesses fast diffusion channels for migration of Na ions and features a two-electron redox reaction mechanism that offers a high theoretical capacity of 170 mA h g−1. However, it is difficult to attain the full discharge capacity of PB in SIBs using the low-spin Fe2+/Fe3+ redox couple. In the present study, we found that doping PB with Ni ions (1–10%) resulted in enhanced electrochemical storage capacity and facilitated fast diffusion of Na ions during discharge. Specifically, PB doped with 3% Ni ions showed a discharge capacity of 117 mA h g−1, within which ≈50 mA h g−1 was attributed to the low-spin Fe2+C6/Fe3+C6 redox couple. Even though we do not know how to attain the full storage capacity of PB, this research sheds light on how substituting transition metal ions affects the electrochemical performance of PB. A new perspective of the electrochemical mechanism is also proposed for further understanding and improvement of its electrochemical performance.


 Please wait while we load your content...
Please wait while we load your content...