Self-supported NiMoP2 nanowires on carbon cloth as an efficient and durable electrocatalyst for overall water splitting†
Abstract
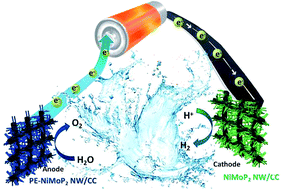
Designing and exploring efficient and stable non-noble bifunctional catalysts by nanostructure modification and chemical composition tuning for water splitting is of critical importance for sustainable resources. Herein, pure phase nickel molybdenum phosphide (NiMoP2) nanowires on carbon cloth are successfully synthesized through a simple and highly reproducible in situ P/O exchange process. Such a NiMoP2 nanowire catalyst requires low overpotentials of 199 and 330 mV to obtain a high current density of 100 mA cm−2 towards the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively, and is among the most active HER and OER electrocatalysts yet reported. The bifunctional NiMoP2 is used as both anode and cathode catalysts in a two-electrode water electrolysis configuration, which delivers a current density of 10 mA cm−2 under a potential of 1.67 V. Furthermore, the overall water-splitting of the bifunctional NiMoP2 nanowire catalyst is further driven by a dry battery with a nominal voltage of 1.5 V which exhibits excellent performance and durability in a strong alkaline electrolyte.


 Please wait while we load your content...
Please wait while we load your content...