Investigation of nano-sized Cu(ii)O as a high capacity conversion material for Li-metal cells and lithium-ion full cells†
Abstract
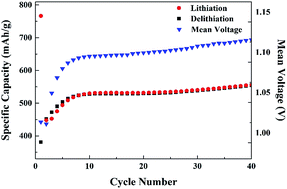
In this study, self-prepared nanostructured CuO electrodes show no capacity decay for 40 cycles at 0.1C in Li metal cells. The reaction mechanisms of the CuO electrodes are investigated. With the help of in situ EIS, in situ XRD, TEM, XAS, SQUID, IC and GC-MS, it is found that the as-prepared CuO electrode undergoes significant phase and composition changes during the initial lithiation, with the transformation of CuO to nano-crystalline Cu. During the 1st delithiation, Cu is inhomogeneously oxidized, which yields a mixture of Cu2O, Cu2−xO and Cu. The incomplete conversion reaction during the 1st cycle is accompanied by the formation and partial decomposition of the solid electrolyte interphase (SEI) as the side reactions. Nevertheless, from the 1st to the 5th delithiation, the oxidation state of Cu approaches +2. After an additional formation step, the transformation to Cu and back to Cu2−xO remains stable during the subsequent long-term cycling with no electrolyte decomposition products detected. The LiNi1/3Mn1/3Co1/3O2 (NMC-111)/CuO full cells show high capacities (655.8 ± 0.6, 618.6 ± 0.9 and 290 ± 2 mA h g−1 at 0.1, 1 and 10C, respectively), within the voltage range of 0.7–4.0 V at 20 °C and a high capacity retention (85% after 200 cycles at 1C).


 Please wait while we load your content...
Please wait while we load your content...