Synthetic manifestation of nitro substituted tetrazole-N-(hetero)aryl derivatives and energetic studies†
Abstract
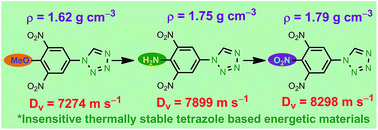
A workable cost-efficient synthetic method for the construction of nitro substituted tetrazole-N-aryl/heteroaryl derivatives is discussed here. The energetic functional groups –NO2, –NHNO2 and –N3 are reliably inserted into the molecular backbone, making the tetrazole-N-aryl derivatives highly energetic and insensitive to heat and impact. For example, the tetrazole derivatives 7 and 8, bearing a –NO2 or a –NHNO2 group, exhibit energetic properties close to RDX, but with enhanced insensitivity. Most of the synthesized compounds show exothermic decomposition and are consequently useful for energetic material applications.


 Please wait while we load your content...
Please wait while we load your content...