Solar-driven Z-scheme water splitting using tantalum/nitrogen co-doped rutile titania nanorod as an oxygen evolution photocatalyst†
Abstract
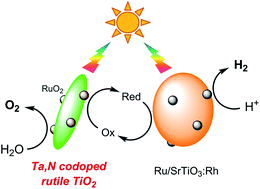
A visible-light-driven water-splitting system that involves two-step photoexcitation (Z-scheme) was constructed using rutile TiO2 nanorod doped with Ta and N (TiO2:Ta/N) as an O2 evolution photocatalyst. The Ta-doped TiO2 nanorods, prepared by a solvothermal synthesis, underwent nitridation to possess visible-light absorption under mild conditions, even at 623 K under an ammonia flow. The TiO2:Ta/N powders modified with a RuO2 cocatalyst were active under visible light up to 540 nm for water oxidation for producing O2 in the presence of reversible electron acceptors (IO3− or Fe3+), while TiO2:N exhibited negligible activity. The results of time-resolved infrared absorption spectroscopy indicated that co-doping Ta with N into TiO2 prolonged the lifetime of photogenerated free electrons, leading to high photocatalytic activity. Simultaneous H2 and O2 evolution via water splitting was achieved using a combination of RuO2-modified TiO2:Ta/N, Ru-loaded SrTiO3:Rh and an Fe3+/Fe2+ redox couple under visible-light irradiation (λ > 420 nm) and under AM 1.5G simulated sunlight.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...