CH3NH2 gas induced (110) preferred cesium-containing perovskite films with reduced PbI6 octahedron distortion and enhanced moisture stability†
Abstract
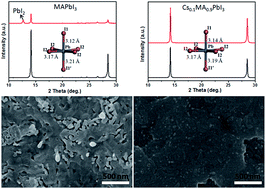
We report here the discovery of a fancy interaction between cesium iodide (CsI) and methylamine (CH3NH2) due to the presence of the hydrogen bond. The formed CsI·xCH3NH2 is a liquid phase, which facilitates the large scale fabrication of highly uniform cesium-containing perovskite films with strong (110) preferred orientation by the CH3NH2 gas healing process. With this method, at most 10% nonpolar Cs cations could fully dope into the crystal lattice and extremely enhance the interaction of the inorganic framework with a more symmetrical PbI6 octahedron, resulting in obvious improvement in moisture stability under continuous illumination.


 Please wait while we load your content...
Please wait while we load your content...