Dynamic covalent bonding-triggered supramolecular gelation derived from tetrahydroxy-bisurea derivatives†
Abstract
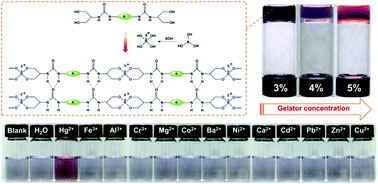
A new class of bisurea derivatives bearing tetrahydroxy groups have been proven to be non-gelators in water and various organic solvents even under long-term sonication or efficient heating treatment. We found that it is possible to trigger physical gelation behaviour by constructing dynamic covalent bonding. The results show that formation of dynamic covalent bonding between the borate anion and ethanediol substituent in these bisurea derivatives brings about rapid physical gelation at ambient temperature in a mixture of DMSO and water. During dynamic covalent bonding-triggered gelation, the stepgrowth polymerization from the B–O bonds would increase the size of the molecules and reduce the entropy of mixing as well as facilitate ion–dipole interactions in the linear polymeric gelators. They would drive a self-assembly transition and boost the construction of gel networks in coordination with α-tape urea–urea hydrogen bonding. The gelation mechanism was explored by 1H NMR, FTIR and rheology techniques. Moreover, the resulting gels are transparent and thixotropic, and could be turned into the sol state under CO2 or water-stimulus. Furthermore, they are stable in the presence of HAuCl4 and alkali. Therefore, they would afford another new medium for the growth of Au nanocrystals via in situ reduction and a new sensing medium for detecting Hg2+ ions.


 Please wait while we load your content...
Please wait while we load your content...