Hydrogen bond directed surface dynamics at tactic poly(methyl methacrylate)/water interface
Abstract
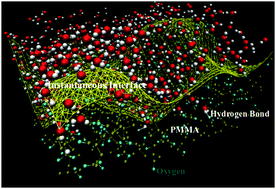
The complexity of induced ordering for tactic poly(methyl methacrylate) (PMMA) thin films in contact with water is examined through all-atom molecular dynamics with validated potentials. We observe that for the water molecules that are hydrogen bonded to the PMMA surface, the isotactic and atactic PMMA show a 33% longer relaxation time compared to syndiotactic PMMA. Almost 94% of hydrogen bonds are with the carbonyl groups of PMMA, irrespective of temperature and tacticity. The stability in re-orientation and nature of hydrogen bond participation for the carbonyl groups as well as about 20% higher interaction energies of carbonyl group hydrogen bonded with water for atactic form indicates existence of cooperative effects. Quantifying the dynamics of hydrogen bond at the tactic interface is important in understanding the role tacticity plays in controlling adhesion and biocompatibility, a design choice that has been gaining ground in the soft material science community.


 Please wait while we load your content...
Please wait while we load your content...