Poly(acrylic acid) interpolymer complexes†
Abstract
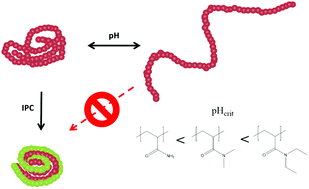
Interpolymer complex formation of poly(acrylic acid) with other macromolecules can occur via several mechanisms that vary depending on the pH. At low pH the protonated acid functional group can form bonds with both donor and acceptor moieties, resulting in desolvated structures consisting of two polymers. Complexes were formed in dilute solutions of PAA, functionalised with acenaphthylene, with a range of other polymers including: poly(NIPAM); poly(ethylene oxide) (PEO); poly(dimethylacrylamide) (PDMA); poly(diethyl acrylamide) (PDEAM) poly(vinyl alcohol) (PVA) and poly(vinyl pyrolidinone) (PVP). Fluorescence anisotropy was used to demonstrate complex formation in each case by monitoring the reductions in segmental motion of the chain as the complexes formed. Considerations of the molecular structures of the complexing moieties suggest that solvation energies and pKas play an important role in complex formation.


 Please wait while we load your content...
Please wait while we load your content...