Self-segregated nanostructure in room temperature ionic liquids
Abstract
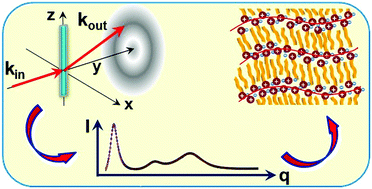
The nanosegregated bulk structure, and its evolution with the cation's alkyl length n, are studied by X-ray scattering for an unprecedentedly broad homologous series of a model room-temperature ionic liquid, [CnMIM][NTf2] (n = 4–22). A tri-periodic local structure is found, with the lateral periodicities, dII and dIII independent of n, and a longitudinal one, dI, linearly increasing with n. The results are consistent with a local structure comprising alternating layers of polar headgroups and apolar, interdigitated, partly overlapping, cations’ alkyl tails, of an average macroscopic mass density close to that of liquid alkanes. A slope decrease in the linear dI(n) suggests a change from a lower to a higher rate of increase with n of chain overlap for n ≥ 12. The order decay lengths of the layering, and of the lateral chain packing, increase with n, as expected from the increasing van der Waals interaction's domination of the structure. The headgroups' lateral packing decay length decreases with n, due to increasing frustration between the longer lateral periodicity preferred by the headgroups, and the shorter lateral periodicity preferred by the chains. A comparison of the bulk and surface structures highlights the surface's ordering effect, which, however, does not induce here a surface phase different from the bulk, as it does in liquid crystals and liquid alkanes.


 Please wait while we load your content...
Please wait while we load your content...