X-ray reflectivity reveals ionic structure at liquid crystal–aqueous interfaces
Abstract
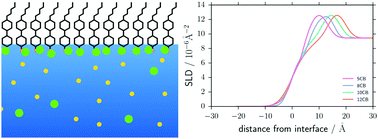
Here X-ray reflectivity has been used to determine the structure of liquid crystal monolayers for different cyanobiphenyl homologues supported on aqueous solutions of two different salt species. Sodium iodide induces homeotropic ordering for all of the monolayer forming liquid crystal homologues studied here, and forms a Stern layer of iodide ions at the liquid crystal cyano headgroup, similar to the case of lipids or surfactants supported on electrolyte solutions. The liquid crystal headgroups were also found to penetrate into the water surface when binding with iodide ions. Sodium bromide, however, does not form the same localisation of ions close to a liquid crystal monolayer, and instead appears to produce no noticeable change in the scattering length density of the liquid crystal monolayer compared to pure water. However, on further compression the X-ray reflectivity dramatically changes, revealing the emergence of the so-called “trilayer” structure for 5CB and 8CB. This transition occurs at a lower areal density for sodium bromide than for pure water, and unlike for the uncompressed film, a layer of bromide ions was found at the trilayer-water interface.


 Please wait while we load your content...
Please wait while we load your content...