Molecular rigidity and enthalpy–entropy compensation in DNA melting
Abstract
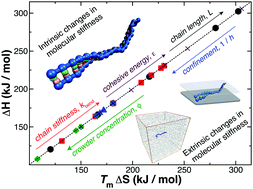
Enthalpy–entropy compensation (EEC) is observed in diverse molecular binding processes of importance to living systems and manufacturing applications, but this widely occurring phenomenon is not sufficiently understood from a molecular physics standpoint. To gain insight into this fundamental problem, we focus on the melting of double-stranded DNA (dsDNA) since measurements exhibiting EEC are extensive for nucleic acid complexes and existing coarse-grained models of DNA allow us to explore the influence of changes in molecular parameters on the energetic parameters by using molecular dynamics simulations. Previous experimental and computational studies have indicated a correlation between EEC and changes in molecular rigidity in certain binding–unbinding processes, and, correspondingly, we estimate measures of DNA molecular rigidity under a wide range of conditions, along with resultant changes in the enthalpy and entropy of binding. In particular, we consider variations in dsDNA rigidity that arise from changes of intrinsic molecular rigidity such as varying the associative interaction strength between the DNA bases, the length of the DNA chains, and the bending stiffness of the individual DNA chains. We also consider extrinsic changes of molecular rigidity arising from the addition of polymer additives and geometrical confinement of DNA between parallel plates. All our computations confirm EEC and indicate that this phenomenon is indeed highly correlated with changes in molecular rigidity. However, two distinct patterns relating to how DNA rigidity influences the entropy of association emerge from our analysis. Increasing the intrinsic DNA rigidity increases the entropy of binding, but increases in molecular rigidity from external constraints decreases the entropy of binding. EEC arises in numerous synthetic and biological binding processes and we suggest that changes in molecular rigidity might provide a common origin of this ubiquitous phenomenon in the mutual binding and unbinding of complex molecules.


 Please wait while we load your content...
Please wait while we load your content...