Thermodynamic stability of worm-like micelle solutions†
Abstract
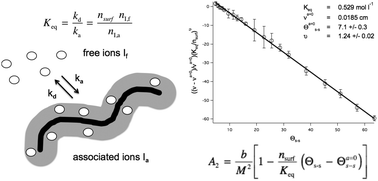
Worm-like micelles (WLMs) are nano-scale self-assemblies widely used for viscosity enhancement, for drug delivery, and in personal care products. The stability of WLMs under variable ionic and surfactant concentrations is important to applications of these fascinating materials. In this work it is demonstrated that a virial approach can be used to understand and predict WLM stability. A mixed surfactant system consisting of sodium laureth sulfate and cocoamidopropyl betaine was examined using small-angle neutron scattering. A linear relationship between the second virial coefficient, A2, and the salt to surfactant ratio, Θs–s, is derived and demonstrated. The Θs–s-dependent term is described via association/dissociation kinetics of salt ions between the bulk and an ion cloud surrounding the WLMs yielding, 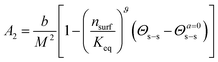 , where Keq is the association/dissociation constant, nsurf is the molar surfactant concentration, b is the molar excluded volume, M the WLM molar mass, Θs–s is the salt–surfactant ratio and Θa=0s–s is the salt–surfactant ratio where the interactions are solely determined by the excluded volume. The ratio b/M2 is independent of WLM contour length. The exponent ϑ is found to be approximately 5/4 in agreement with polymer scaling laws for the semi-dilute regime in good solvents.
, where Keq is the association/dissociation constant, nsurf is the molar surfactant concentration, b is the molar excluded volume, M the WLM molar mass, Θs–s is the salt–surfactant ratio and Θa=0s–s is the salt–surfactant ratio where the interactions are solely determined by the excluded volume. The ratio b/M2 is independent of WLM contour length. The exponent ϑ is found to be approximately 5/4 in agreement with polymer scaling laws for the semi-dilute regime in good solvents.


 Please wait while we load your content...
Please wait while we load your content...