Mechanism of ion transport in perfluoropolyether electrolytes with a lithium salt
Abstract
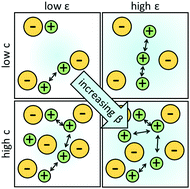
Perfluoropolyethers (PFPEs) are polymer electrolytes with fluorinated carbon backbones that have high flash points and have been shown to exhibit moderate conductivities and high cation transference numbers when mixed with lithium salts. Ion transport in four PFPE electrolytes with different endgroups was characterized by differential scanning calorimetry (DSC), ac impedance, and pulsed-field gradient NMR (PFG-NMR) as a function of salt concentration and temperature. In spite of the chemical similarity of the electrolytes, salt diffusion coefficients measured by PFG-NMR and the glass transition temperature measured by DSC appear to be uncorrelated to ionic conductivity measured by ac impedance. We calculate a non-dimensional parameter, β, that depends on the salt diffusion coefficients and ionic conductivity. We also use the Vogel–Tammann–Fulcher relationship to fit the temperature dependence of conductivity. We present a linear relationship between the prefactor in the VTF fit and β; both parameters vary by four orders of magnitude in our experimental window. Our analysis suggests that transport in electrolytes with low dielectric constants (low β) is dictated by ion hopping between clusters.


 Please wait while we load your content...
Please wait while we load your content...