Electrolyte effect in induced charge electroosmosis†
Abstract
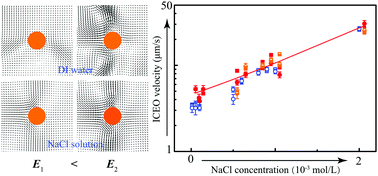
This paper reports an interesting phenomenon that the velocity increases with increasing sodium chloride (NaCl) concentration in induced charge electroosmosis (ICEO) around a conducting cylinder measured by microparticle image velocimetry (μPIV). It is different from the widely reported velocity decay with increasing electrolyte concentration in AC electroosmosis (ACEO) [M. Z. Bazant et al., MicroTAS, 2007, 2875–2878] and induced charge electrophoresis (ICEP) of Janus particles [S. Gangwal et al., Phys. Rev. Lett., 2008, 100, 058302]. In addition, it is found that a reversed vortex flow emerges in deionized water. As the electric field increases or with a slight addition of NaCl, the ICEO vortex flow recovers. Different from the prediction of thin electric double layer (EDL) models, the observed ICEO flow is asymmetric with respect to the cylinder center. The asymmetry presents an electrolyte dependence, and it is intensified as the applied electric field increases. The ICEO flow obtains maximum values at certain electric field frequencies in NaCl and calcium chloride (CaCl2) solutions. But it is surprisingly insensitive to the electric field frequency in sodium dodecyl sulfate (NaDS) solutions. The optimum electric field frequency varies as electrolyte species change. A linear relationship between ICEO velocity and electric field strength squared is observed in all the examined electrolyte solutions, while the slope varies with the changing electrolyte species.


 Please wait while we load your content...
Please wait while we load your content...