Synthesis and organogelating behaviour of amino acid-functionalised triphenylenes†
Abstract
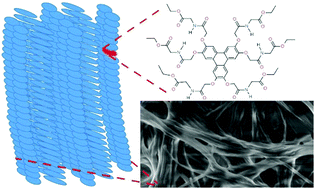
Four novel amino acid-functionalised triphenylenes have been prepared with glycine, L-alanine, L-phenylalanine and L-tryptophan ethyl ester side-chains. The glycine derivative is a good gelator of chloroform, the alanine derivative gels ethanol and toluene, and the phenylalanine derivative gels benzene and toluene. The tryptophan derivative does not gel any of the solvents tested, most probably due to its more bulky structure, but forms microspheres by evaporation-induced self-assembly. The self-assembly properties of the π-gelators have been investigated using infrared, UV-absorption and fluorescence spectroscopy, concentration- and temperature-dependent NMR, and X-ray scattering experiments on dried xerogel as well as the wet organogel. The latter experiments suggest the glycine gel in chloroform includes columnar aggregates, with an overall disordered columnar oblique mesophase. These compounds are of interest because of the well-known hole-transporting properties of triphenylene liquid crystals: 1-D columnar assemblies of these compounds may find applications in organic electronic devices.


 Please wait while we load your content...
Please wait while we load your content...